Quality by Design
Main
Quality by Design (QbD) in the pharmaceutical and biopharmaceutical industries is a systematic approach to development of drug products and drug manufacturing processes that begins with predefined objectives, emphasizes product and process understanding and sets up process control based on sound science and quality risk management. Under the QbD paradigm, pharmaceutical quality is assured by understanding and controlling manufacturing and formulation variables. End product testing is used to confirm the quality of the product and is not part of the ongoing consistency assurance and/or process control. The QbD framework is based on the ICH Guidelines that define QbD for both the pharmaceutical and biopharmaceutical industry (ICH Q8-Q11).
Process understanding allows our manufacturing to move from requesting changes by the FDA to reporting that a change will occur based on the process design space. Better practices in product and process development have the potential to raise the profits of pharmaceutical companies based on product and process development. The ability to define the association between product parameters and product development processes early in the development lifecycle will in turn have a high impact on the eventual manufacturing efficiency.
QbD Roadmap
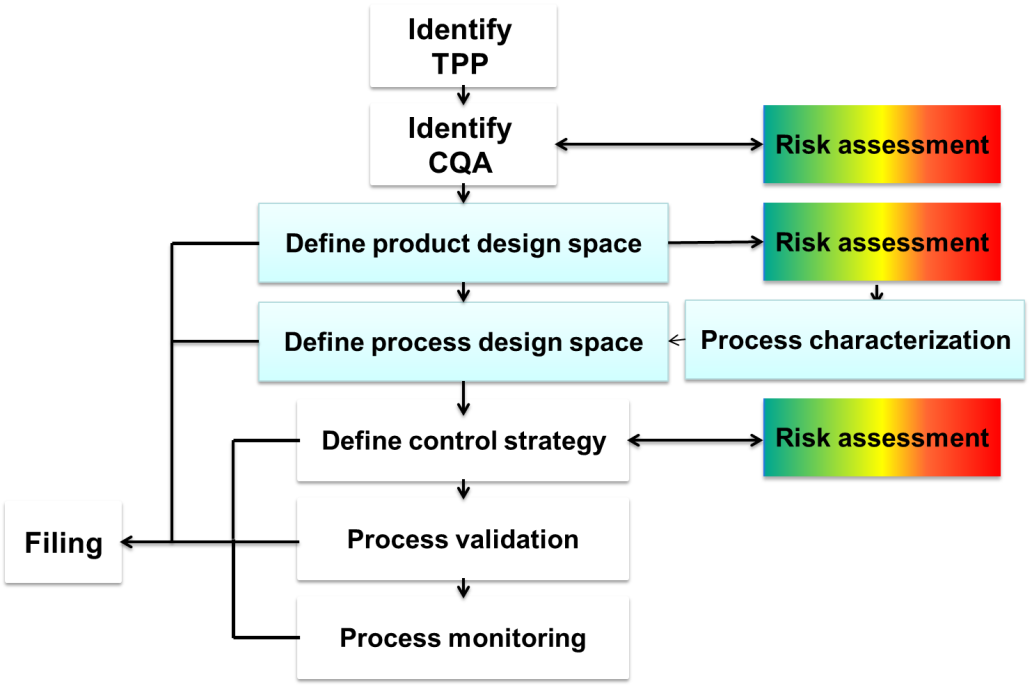
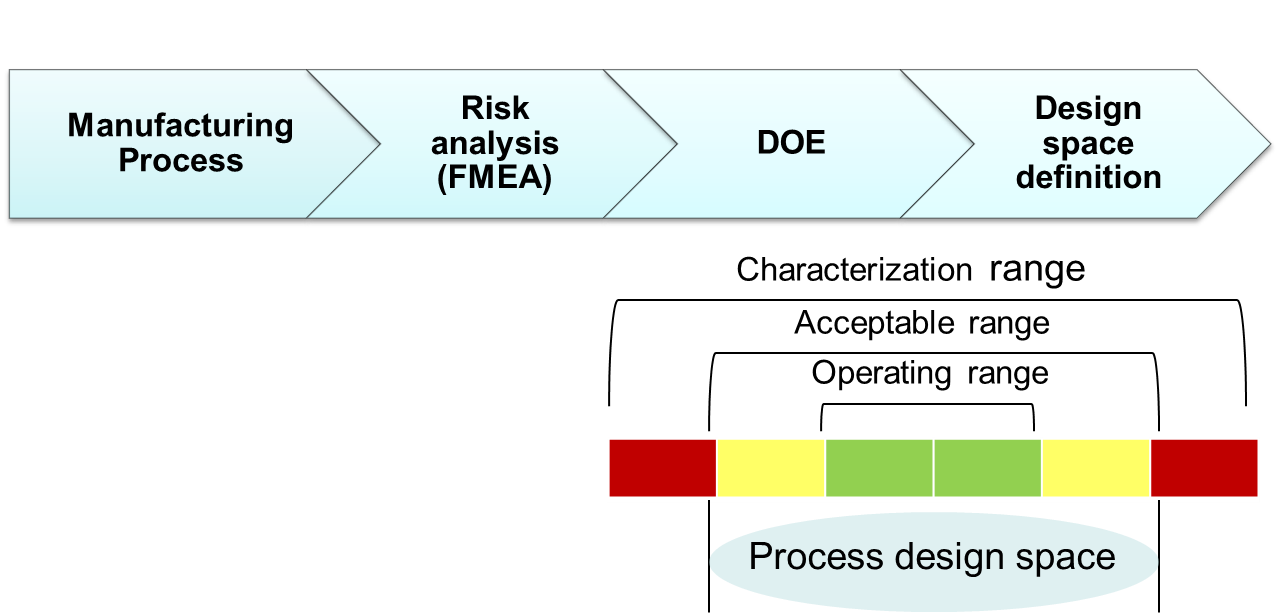
Questions we seek to answer during the QbD initiative:
- What sources of variation are present in the system?
- Can we use the data-derived information to assess risk and uncertainty?
- How are we using our integrated process understanding to determine and verify the way we control our processes?
- As we evolve and stabilise our processes are we continually evaluating the risk around our control strategy?
Benefits of QbD:
- Minimize manufacturing cost and risk
- Improved manufacturing efficiency and scale up
- Increase profits through a reduction in annual COGS and R&D spending
- Ability to report updates within the manufacturing process to the FDA without a requirement for approval
What activities and responsibilities are part of KPA offering?
KPA guides pharmaceutical and biopharmaceutical companies in the development and implementation of the QbD roadmap based on the industry guidelines ICH Q8-Q11. Our process begins with general lectures on QbD to align all team members on the QbD initiative followed by practical projects with team members from product and CMC. During the practical project phase, KPA will support the team members during all stages of QbD implementation including Risk assessment, QTPPs identification, CQAs identification, product and process design space, control strategy and raw material management. During this stage the tech transfer of QbD concepts and methodologies will take place. During each stage of implementation KPA will utilize statistical approaches and tools for risk management, DoE and multivariate statistics. Our goal is to establish acceptable boundaries of critical quality attributes to define a design space for a given process step.
What are the main deliverables/achievements?
KPA guides and contributes to the development of the QbD roadmap and works directly with your product and CMC teams to implement QbD. The QbD process is continuous with an added value gained through operational efficiency, reduction in compliance remediation costs and improved product development.
What is unique regarding KPA offering or service?
KPA contains extensive experience in QbD implementation and biostatistics. Our consultants and statisticians have a wide range of expertise covering Quality Risk Management and Risk Assessment, Design of Experiments (DoE), Mixture DoE, Stability Analysis and Statistical Methods for Process Validation as touch points for the QbD roadmap within the pharmaceutical and biopharmaceutical industries.
We have the knowhow and expertise to guide and work with your team in achieving the vast benefits of Quality by Design.


